|
|
|
|
|
|
Direct Energy Conversion from Hydrocarbon Fuels using
Electromagnetic Waves
|
|
|
|
Energy of hydrocarbon fuels converted directly from chemical form to
electrical form
|
|
|
|
Patent application no. - 336/BOM/99
|
|
|
|
A C - H bond is formed by mutual sharing of an electron from carbon
item and hence no
electron is released when the bond breaks, energy is
released in the form of heat.
To break a molecular bond, some energy is required which is done by
igniting the fuel. The energy provided is activation energy. When
activation energy is provided to the fuel molecule, the C- H bond
breaks and releases heat energy.
When a bond breaks the electron shared by carbon and hydrogen atom
goes back to the carbon atom. An electron goes into lower orbit of
the atom, which is the lowest energy state. The electron shared in
covalent bond does not have energy sufficient to
escape from the atom, hence electron emits the extra energy and
jumps to the lower Energy State. Therefore the hydrocarbon fuel
energy is normally extracted in the form of heat by burning the fuel.
|
|
| |
Invention (What is New) |
|
|
|
To extract energy from hydrocarbon fuel in the form of electricity,
the shared electron in C - H bond should have sufficient energy so
that it can escape from atom leaving behind an H+ ion. (the free
electron and H+ ion form an electric current source). To make an
electron jump out of C - H bond, sufficient amount of energy is
required to be provided from outside. (catalytic quantum ) The
catalytic quantum of energy provided externally makes an electron
'stronger ' to escape from the hydrogen atom. If this is achieved,
the electron has energy that is equal to sum of bond energy and the
catalytic quantum (Energy of an electron =
Catalytic quantum + Bond energy). The catalytic quantum is
re-used to extract bond energies as the catalytic quantum used for the next
cycle is
generated from the energy extracted.
|
|
|
|
Diagram & Description |
|
|
|
Ionization energy for an isolated H atom is 1310 kJ / mole. for one
H atom, ionization energy is 2.1749 x 10-18J/
atom. When hydrogen forms a bond with carbon atom, the shared
electron has some energy in the bond.
The energy in one C -H bond is 0.3699 x 10-18 J
/ bond .
To ionize the H atom, the amount of external energy (catalytic
quantum) is provided which is equal to the difference between the
bond energy of C - H bond and ionization energy of the H atom.
Ionization
energy - Bond energy = Catalytic quantum
(2.1749 x 10-18) J/ atom - (0.3699 x 10-18) J/
CH bond = 1.805 x 10-18J/ atom
Hence, the external energy to be provided is 1.805 x 10-18 J
/ atom.
i.e. per C-H bond since each bond has one hydrogen atom.
For energy provided in the form of electromagnetic waves. The
frequency and wavelength of the electromagnetic wave is,
E= hf, (E= energy of wave, h= Plank's constant, f= frequency of
wave.)
J
= 6.625 x 10x
frequency of wave
Hence ,
f= 2.7 x 1015 cycles / second
wavelength = c/f, (c = speed of electromagnetic wave = 2.97 x 10m/s)
wavelength is 1.090 x 10 m
= 1090o A.
An electron in C - H bond has only 17% (0.3699 x 10-18)
of Ionization energy Hydrogen atom. Remaining 83%
(1.805 x 10-18 J
) energy is provided externally in the form of electromagnetic
waves.
Energy in C-H bond + catalytic quantum = Ionization energy + (H+
ion)
|
|
|
|
Working |
|
|
|
The chemical energy stored in Carbon-Hydrogen bonds of liquid
hydrocarbon fuel molecule is converted into electrical energy. The
liquid hydrocarbon fuel is exposed to collimated electromagnetic
waves in absence of air. The wavelength of incident collimated
electromagnetic wave is 1090o A units(1090 x 10-10 m). The
electromagnetic wave transfer its energy to the electron in the
chemical bond between Carbon and Hydrogen. The electron absorbs the
energy and jumps out of the hydrogen atom.
The Hydrogen atom becomes a positive Hydrogen ion. And the electron
becomes a free electron. The liquid fuel is kept in a magnetic field
while it is exposed to electromagnetic waves. The magnetic field
directs these free electrons towards a metal plate, that acts as a
metal electron collector and as an electrode. The positive Hydrogen
ions are directed towards a synthetic membrane (proton exchange
membrane). The synthetic membrane passes only positive Hydrogen ions
through it. While passing through the membrane, positive Hydrogen
ion gains an electron from the membrane and combines with Oxygen
atoms in the air which is on the other side of the membrane (the
Hydrogen ion gives it's positive electrical charge to the membrane).
The membrane, which also acts as an electrode, gets positive
electrical charge. The metal electron collector becomes
negatively charged due to excess electrons collected on it. The two electrodes are connected through an electrical
circuit. An electrical current flows through the circuit, which is
energy source.
Part of the electrical energy is consumed to generate
electromagnetic waves that are used for energy conversion and
remaining part of energy is available for use.
|
|
|
|
IN THE DRAWING A
'1 ' is collimated electromagnetic wave source.
'2' is fuel molecule.
'3' is proton exchange membrane that passes only positively charged
Hydrogen
ions.
'4' is a metal electron collector.
'5' are permanent magnets .
'6' is a fuel container .
'7' is a waste Carbon collected at the bottom of container.
'8' is a continues airflow.
'9' is a water molecule formed at the outer side of the membrane .
'10' is Minimum fuel level.
'11' is a battery in which the converted electrical energy is stored
.
'12' is liquid hydrocarbon fuel.
‘13’ is collimated electromagnetic wave.
|
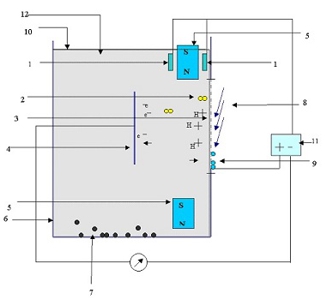
Drawing A |
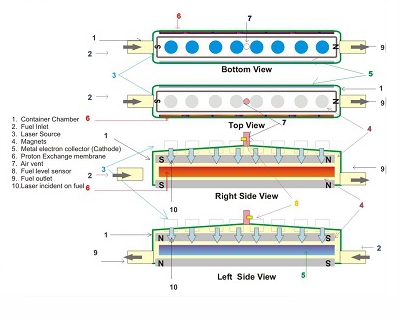
Conceptual view of device with flowing fuel |
|
|
Calculation |
|
|
|
1. For CH4 i.e. , the caloric value is 55700 kJ/ Kg.
Molecular weight of methane ( CH4 ) is { (12 x 1) + ( 1 x 4
) } = 16
Since CH 4 delivers 55700 kJ/ Kg = 55700 J/g
One mole of methane delivers 55700 J x 16 =
891200 J/ mole.
Each mole contains 6.023 x 1023molecules,
Energy delivered per molecule is = (891200 / 6.023 x 1023)J
/ molecule . = 1.4796 x 10-18J
/ molecule.
Each molecule of CH 4 has four C – H bonds
Therefore one C- H bond in methane has energy ( 1.4796 x 10-18)
/ 4 = 0.3699 x 10-18J/
C – H bond.
When one C-H bond breaks, the amount of energy
released is 0.3699 x 10-18J
------------------------- I
2. The ionization energy of an isolated Hydrogen atom is
1310 kJ / mole = 1310000 J / mole
Hence ionization energy per atom is (1310000 /
6.023 x 1023)
J / atom = 2.1749 x 10-18J
/ atom ------------------------II
From I …… one C-H bond has energy equal to
0.3699 x 10-18
From II……. one-H atom needs 2.1749 x 10-18J
to get ionized.
When C-H bond breaks, to ionize H atom (to extract an
electron from H atom) an amount of energy to be provided
from outside is equal to the difference between ionization
energy of isolated Hydrogen atom and the energy stored in
the C - H bond.
catalytic energy quantum = ionization energy –
C-H bond energy = (2.1749 x 10-18J/C-H
bond) - (0.3699 x 10-18J/C-H
bond) = 1.805 x 10-18J/C-H
bond.
The energy provided from outside enables the electron
(shared by carbon and hydrogen atom in C-H bond) to escape
from the H atom leaving behind an H+ ion and carbon. The
electron and an H+ ion are used to form an electric current.
The external energy (catalytic quantum) is provided in the
form of electromagnetic waves where energy of wave = 1.805 x
10-18J
Since ,
Energy = h x frequency of wave, (h = Plank’s constant = 6.625
x 10-34
)
1.805 x 10-18 J = 6.625 x 10-34 x
frequency
frequency = 2.724 x 1015 cycles per second.
speed of an electromagnetic wave is 2.97 x 108 m
/s
the wavelength of the wave = ( 2.97 x 108/
2.724 x 1015)
m. = 1090 x 10-10=
1090 0 A.
Following are the details of the energies for
different fuel quantities
1
gm. octane delivers energy of 35170 J by breaking C-H bonds
Catalytic
quanta required is 171650 J
It
generates 206824 J in the form of electricity (catalytic
quanta + C-H bond energy)
Total electrical energy - Catalytic quanta = Energy
available for use.
To generate 1 kW energy the catalytic energy quanta needed
is (171650 / 35170) J = 4.8796 times the energy needed i.e.
4.8796 kW
Thus catalytic quanta to be provided is 4.8796 times the
amount of energy needed from octane
|
|
USES |
|
This conversion process is useful for automobiles where liquid hydrocarbon fuel is used.
Automobiles use internal combustion engines to generate kinetic energy from
fuels. The losses involved in internal combustion engines
are about 60%.
The Direct Energy conversion
system for hydrocarbon fuel would provide a better option to
an Internal Combustion Engine, thus avoiding energy losses
and pollution.
|
|
|


